Due Date: May 6, 2024
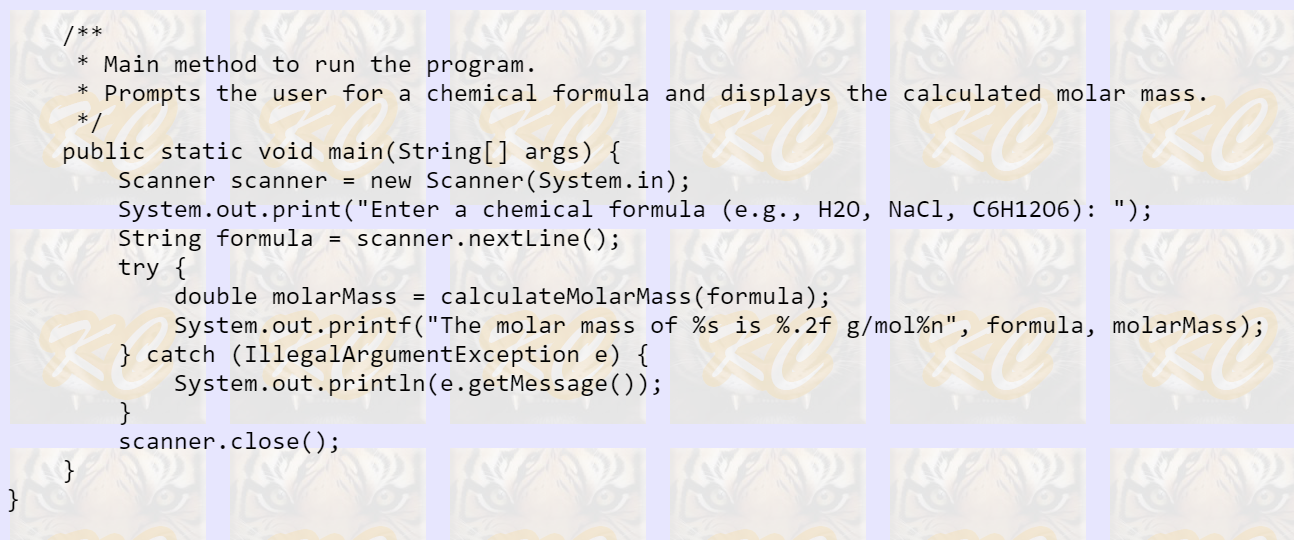
Java program to calculate Molar Mass
How to Calculate Molar Mass of a Chemical Compound
To calculate the molar mass of a chemical compound:
1. Identify the Formula: Note the compound's molecular formula, e.g., H2O.
2. Get Atomic Masses: Use a periodic table to find
the mass of each element in grams per mole (g/mol).
3. Count Atoms: Count how many atoms of
each element are in the formula.
4. Calculate: Multiply each element's
atomic mass by its count, then sum these values.
For instance, water (H2O) has a molar mass
of 18.016 g/mol, calculated as (2 x 1.008) + 16.00.
To learn more about calculating Molar Mass click here.
Your files will be:
PX_MolarMass_lastname.java (Actual Java program)
PX_MolarMass_lastname.png (Screen shot of the
program in the Eclipse IDE)
PX_MolarMass_lastname.mp4 (Video)
(Video should include an explanation of the program
and showing it running successfully)
Be sure to drop these files into google classroom.
Click here to read the specs for this program.
Click here to read the Pseudocode for this program.
Click here to learn about HashMap with examples.
(You will use the HashMap in this assignments.)
I have the program code below with a section of code missing.
**** Section 1 *****
 **** Section 2 *****
**** Missing code ****
**** Section 3 *****
**** Section 2 *****
**** Missing code ****
**** Section 3 *****
 **** Section 4 *****
**** Section 4 *****




 **** Section 2 *****
**** Missing code ****
**** Section 3 *****
**** Section 2 *****
**** Missing code ****
**** Section 3 *****
 **** Section 4 *****
**** Section 4 *****
